Project
Approaches
High-resolution metabolomics
High-resolution metabolomics (HRM) has been developed to provide a high-throughput platform capable of detecting chemicals used for standard clinical measures (such as glucose, creatinine, amino acids, bilirubin, cholesterol, urea), environmental chemicals (such as parent chemicals and metabolites) and assessing biological response to internal and external stressors (for example, presence of prostaglandin molecules suggest inflammatory response). The comprehensive metabolic information obtained from such an analysis enables a more thorough characterization of an individual’s metabolic phenotype, which can be defined as all endogenous biological metabolites, the chemicals from human–environment interaction, and reactants arising from interaction of these compounds with enzymatic and bacterial processes occurring within multiple body components. Easily accessible biological fluids, such as blood and urine, are used to define the metabolic phenotype. Measurement of an individual’s metabolic phenotype has many applications in precision medicine, population screening, chemical monitoring and for understanding the pathophysiology of diseases. Importantly for this project, the metabolic phenotype can be assessed for the presence of exposure and effect biomarkers arising from environmental exposures. By applying HRM based upon liquid chromatography separation and high-resolution mass spectrometric detection, we will attempt to measure as many environmental chemicals as possible for use as functional inputs in environment wide association study of ASD.
Liquid chromatography
The HRM platform developed in the Clinical Biomarkers Laboratory at Emory University uses liquid chromatography (LC) for separation of chemicals prior to detection. Unlike gas chromatography, which requires chemical species to be volatile (able to easily leave solution and form a gas at certain temperatures), LC uses solubility in liquid solvents, such as water and acetonitrile, to separate different species from each other. In addition, many compounds found in blood and urine are polar and prefer to remain in solution, which is an important consideration when wishing to avoid chemical or physical modification of structures prior to analysis. While using a separation technique before detection increases the time required to analyze samples, it plays an important role in HRM. Oftentimes, biological specimens contain high concentrations of chemicals (for example, sodium and other salts) that can interfere with the detection of other species present at very low levels. The use of LC can avoid this type of interference (referred to as matrix effects) by retaining analytes of interest so other, less relevant compounds can elute. Another advantage is the ability to separate chemicals that have identical chemical formulas, but different chemical structures. Pairs of such chemicals are called isomers, and will be present with identical masses but different elution times from LC columns.
The principle processes involved in LC include sample preparation, injection, column separation and analyte detection. Sample preparation is completed to make the sample consistent with the liquid phase flowing through the column (called the mobile phase or eluent) and remove large chemicals such as proteins, nucleic acids and biopolymers. Following sample preparation, the extract is stored in a refrigerated sampler that allows automated injection onto a column where the LC separation will take place. The column itself consists of a cylindrical steel tube that is typically 5 to 10 cm long and packed with small particles covered with a chemical coating (called the stationary phase). Many different stationary phase options are available, and the type selected will control the time different species of chemicals are retained in the column (defined as the retention time). The most commonly used separation scheme is called reverse phase chromatography (RPC) and utilizes a stationary phase consisting of octadecyl carbon chains (C18) bonded to silica particles. In an RPC based separation, the initial mobile phase conditions are such that the majority of the analytes have a greater affinity for the stationary phase than the mobile phase, which will result in the individual molecules collecting within the C18 coating. After a set amount of time that allows for washing out of non-retained interferences, a transition from aqueous dominated mobile phase to organic solvent dominated is completed, resulting in favorable mobile phase conditions for release of C18 retained molecules. Often times, a weak acid such as formic acid or acetic acid is present to buffer mobile phase pH. Based upon physicochemical properties, release will occur at different organic solvent fractions, resulting in the presence of analyte peaks over the course of the run. Typically, small, polar metabolites elute early (such as amino acids, carnitines, carbohydrates, environmental chemical metabolites), small, non-polar metabolites elute during the middle of the run (steroids, hormones, fatty acids, environmental chemicals) and large, non-polar metabolites elute late (lipids). Following completion, LC columns can be washed, re-equilibrated and used for subsequent injections.
Go to top!
High-resolution mass spectrometry (HRMS)
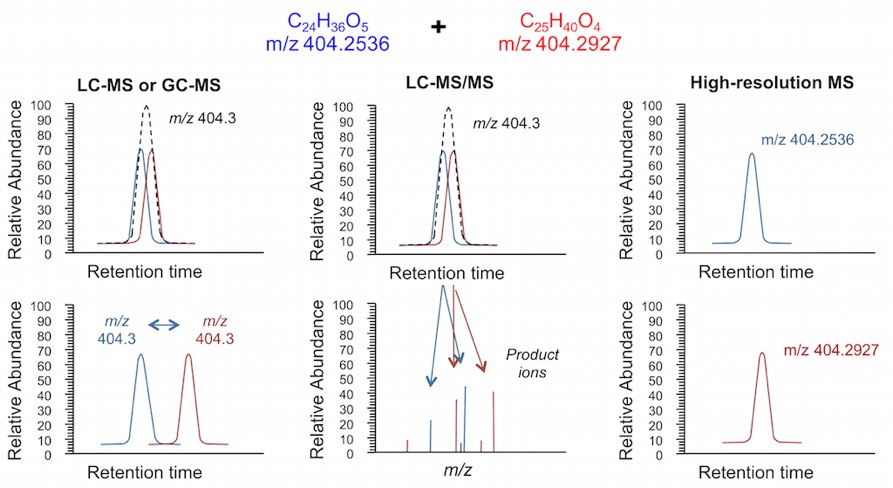
While LC provides separation of different chemical species, primary advances in high-resolution metabolomics has been achieved with mass spectrometers capable of providing ultra-high mass accuracy measures of low abundance chemicals. In the Clinical Biomarkers Laboratory, Fourier transform mass spectrometers with a specialized Orbitrap chamber are used to measure chemicals present in biological samples. Specific models include the Thermo LTQ Velos, Fusion and Q-Exactive HF. To understand the advantages of such instruments, it is important to consider what is being measured by mass spectrometers and how detected signals, including mass-to-charge ratio (m/z) and intensity, can be used in metabolic phenotyping studies.
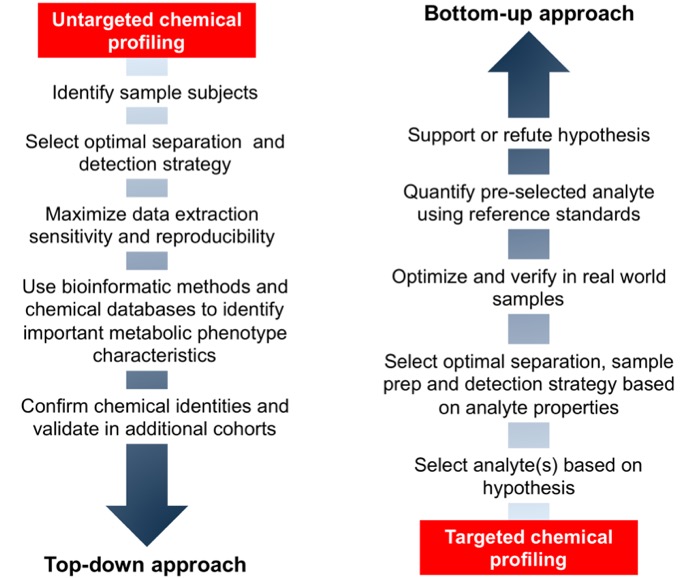
Mass spectrometers are based upon measurement of gas-phase ions in a vacuum. When connected to a LC system, the most common mechanism for formation of ions is solvation of liquid droplets in an electric field and subsequent ionization of analyte molecules. The ions generated are referred to as adducts, and most often consist of the original chemical structure plus addition (positive ionization) or loss (negative ionization) of a proton (H, +1.007276 dalton). Formation of the adduct results in a molecule with a net positive or negative charge, which is required since mass spectrometers can only detect ions and not neutral molecules. The ion is detected as the m/z ratio, with intensity proportional to the concentration in the sample. Since mass is an intrinsic property of a molecule, it is possible to selectively detect analytes, even when other chemicals are present at the same retention time, as long as they are not isomer. Furthermore, since common mass additions during ionization are known, possible identities of detected signals can be determined by comparing the calculated adduct mass for known chemicals to detected m/z values. For example, phenylalanine, an essential amino acid with a mass of 165.0790, will routinely form a +H adduct that can be detected with a m/z value of 166.0863. The ability to detect analytes as distinct m/z values for defining chemical identity and concentration are the primary advantages of HRMS (Fig. 1). In contrast, most laboratories use unit-based instrumentation, which is only able to measure chemicals within a mass range of 1 dalton. The high accuracy available from HRMS instruments often allows identification based on the m/z values alone, an important advantage when trying to detect a large number of chemicals that are often not known beforehand. The main differences between untargeted (referred to as top down approaches) and targeted (bottom up approaches) are shown in Fig. 2. In this project, we are combining both to try and measure as many chemicals as possible to identify environmental risk factors of ASD. The untargeted analysis will be completed using the LC-HRMS methods described above, while the targeted analysis will be completed using the GC-MS platform described here. Details on the chemicals measured using the untargeted methods are described below.
Chemical coverage
Central metabolic pathways that are essential for life include approximately 2000 metabolites produced within your body. In addition to these chemicals, referred to as endogenous metabolites, a more broader view of chemicals present within your body include those from the diet, microbes present in your gut and chemicals that are present due to the environment. Chemicals arising from the external environment are called exogenous chemicals, and measuring their levels in human populations is referred to as biomonitoring. Examples of chemicals we can measure include:
- Nutrient and intermediate metabolites: Carbohydrate metabolism, amino acid metabolism, nucleotide metabolism, cofactor metabolism, steroid metabolism, lipid/fatty acid metabolism, terpenoid metabolism, nutrients, additional secondary and amino-group metabolism
- Dietary and food-related metabolites: Phytochemicals, essential amino acid metabolism, diet-related biomarkers, fatty acid metabolism, supplements
- Pharmaceuticals: Non-steroid anti-inflammatory drugs, platelet aggregation inhibitors, analgesics, antitussives, acetaminophen, anticonvulsants, antihistamines, bronchodilators, anesthetics, vasodilators
- Environmental chemicals: Phosphoester flame retardants, carbamate pesticides, phenylurea pesticides, neonicotinoid insecticides, phthalates, pesticide synergists, thiocarbamates, chloroacetanilide herbicides, organophosphates
The ability to measure exogenous chemicals and their metabolites, which can be used to estimate environmental exposures, is the reason we are applying LC-HRMS techniques to profile blood samples from children with ASD, siblings, parents and controls. We typically detect 15,000 to 20,000 different chemical signals in blood samples obtained from humans in the United States. We hope that applying such expansive measurements will help us identify environmental risks contributing to ASD.
Definitions:
Metabolic phenotype – Metabolic chemical profile measured within a biological sample using a variety of analytical platforms. The metabolic phenotype will includes endogenous metabolites, dietary chemicals, pharmaceutical drugs, environmental chemicals, microbiome related chemicals and all metabolites originating from interaction of these chemicals with host and environmental processes.
Liquid chromatography – A separation approach useful in the analysis of non-volatile chemicals.
Matrix effects – Influence of additional components on the detection and quantification of a given analyte, in this case referring to analysis by mass spectrometry.
Isomer – tTwo or more molecules with the same chemical formula but different chemical structures.
Mobile phase (eluent) – Liquid phase driving interaction with the stationary phase during LC separation. Typically consists of buffer, water and organic solvent (such as methanol or acetonitrile).
Stationary phase – Chemical coating grafted onto a solid support structure within LC columns that interacts with analytes during LC separations.
Retention time – Time required for a chemical to pass through an LC column, driven by analyte physiochemical properties, stationary phase and composition of the mobile phase.
Reverse phase chromatography – Popular separation technique that uses chemical affinity for polar and non-polar phases to separate chemicals in a solution.
High-resolution mass spectrometry – Mass spectrometers that are capable of differentiating between different masses with very fine resolution (0.0005-0.001 dalton). Examples include Fourier transform ion cyclotron, Orbitrap and quadrupole time-of-flight mass spectrometers.
Mass-to-charge ratio (m/z) – Chemicals ionized by the mass spectrometer are detected as the mass of the analyte adduct divided by the charge of the ion. Analytes with a single charge will be detected as the adduct mass divided by one, doubly charged adducts will be measured as the adduct mass divided by two, etc. For low molecular weight chemicals, singly charged ions are most common.
Intensity – Area under the curve for a given m/z signal. The intensity is proportional to the amount (concentration) of the chemical present in the biological sample.
Adduct – Chemical species formed during electrospray ionization that causes the analyte molecule to become positively or negatively charged.
Endogenous metabolites – Chemicals that originate within a host organism.
Exogenous chemicals – Chemicals that do not originate within a host organism. Can include chemicals from diet, pharmaceuticals and environmental exposures. The presence of exogenous chemicals does not necessarily indicate an adverse health effect. For example, nutrients are beneficial but can be considered exogenous in origin since they are obtained from the food we eat.
Biomonitoring – The process of measuring environmental chemicals or markers of their presence in biological samples.
Go to top!
Please contact douglas.walker@emory.edu with any questions.