Project
Approaches
GC-MS
Gas chromatography and mass spectrometry (GC-MS) is an analysis technique which allows for measurement of a mixture of chemicals at very low concentrations. The analysis is conducted in two steps: separation and detection. GC-MS is used for many applications, including illegal drug screening, doping analysis in sports, detection of pesticides in foods, and detection of explosive compounds for airport security.


Separation—Gas Chromatography
Gas chromatography (GC) is a process by which chemicals are separated based on their physical and chemical properties. The sample is vaporized and injected into a long thin polymer-coated glass tube (called a GC column) with gas flowing through it. The column is stored in an oven, which allows heating of the sample to aid vaporization. As the sample vapor is carried through the column, various chemicals in the sample have a stronger tendency to “stick” to the polymer coating, and thus take longer to pass through. At the end of the column, the separated chemicals will exit the column and be detected in pulses, or chromatographic peaks.
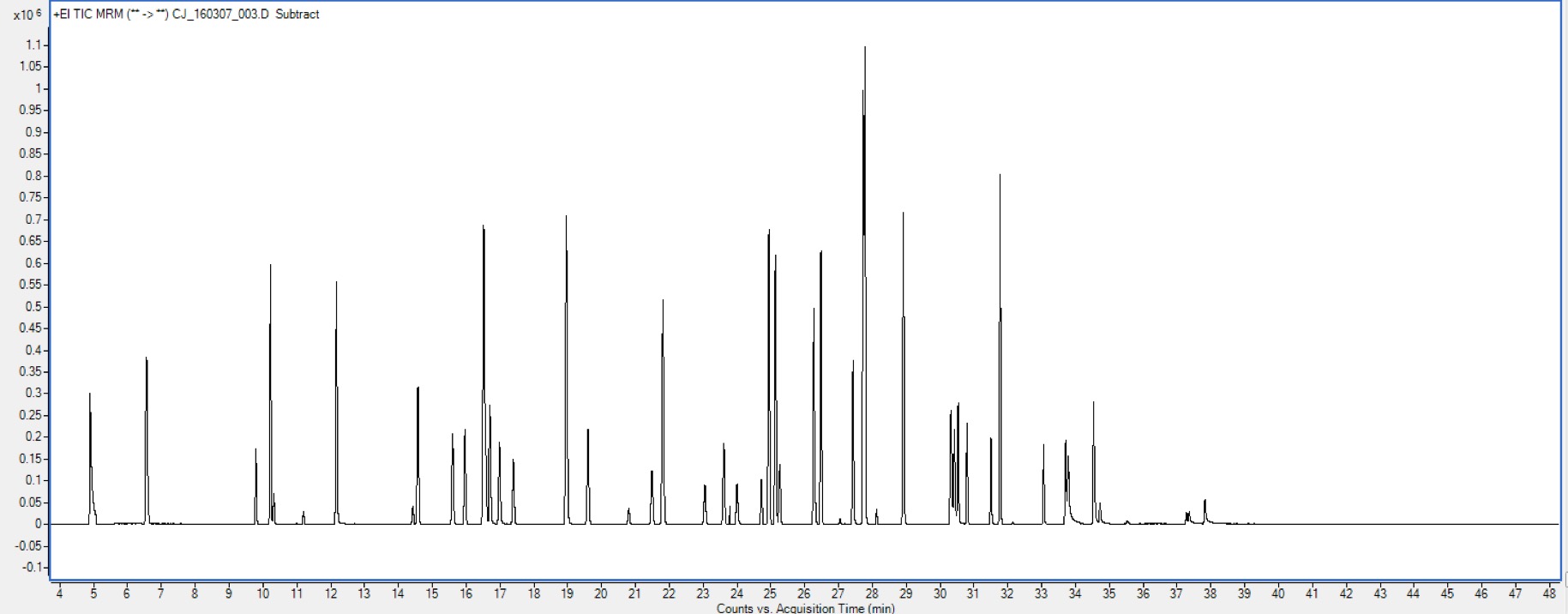
Detection—Mass spectrometry
Mass spectrometry (MS) is a type of detector that can be placed at the end of a GC column to detect the chemicals after they are separated. While other detectors may detect chemicals by thermal conductivity, combustion energy, or electronegativity, MS measures compound masses. Within a mass spectrometer, chemicals are bombarded with high energy radiation until they are fragmented, or broken into smaller ions. These ions are then measured by a mass selective detector to produce a mass spectrum, which is unique for each chemical. This mass spectrum allows chemicals with different mass to be analyzed separately, even if they exit the GC column at the same time (co-elution).

Improving Detection—MS/MS
A MS detector can only measure a certain amount of ions at once. In addition, sometimes ions from unidentified chemicals in the sample can obscure parts of the mass spectrum, making it hard to identify. At the low concentrations at which environmental contaminants are often measured, even the slightest bit of such interference can present a problem. To overcome this difficulty, a technique called tandem mass spectrometry, or MS/MS, is used to provide even more selective detection. In MS/MS, one ion is selected from a chemical’s mass spectrum. This ion, the precursor ion, is bombarded with energy again to create smaller fragments known as product ions. A precursor and product ion together form a mass transition, and most compounds can be identified uniquely by measuring only two different mass transitions. This unique measurement virtually eliminates “noise” from unknown or irrelevant compounds, and thus it allows for detection at very low concentrations.
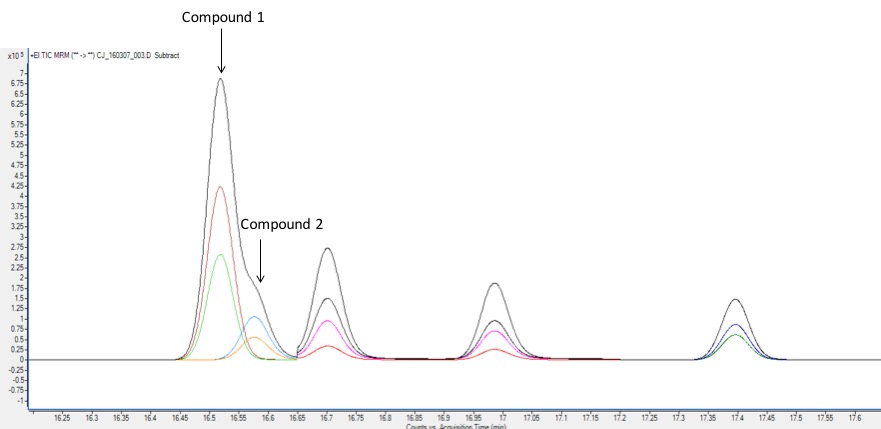
Why is GC-MS/MS important to this project?
The broad range of possible environmental exposures means that it is important to measure as many chemicals as possible. Using GC-MS/MS, we can measure hundreds of compounds in one analysis, allowing us to better characterize participants’ exposures. Human exposure is measured using plasma, blood, or urine samples which can contain millions of different chemicals, and thus GC-MS/MS is also preferred because only the compounds of interest are measured.
Definitions:
chromatographic peaks – areas of increased detector signal that occur when a compound exits the GC column
mass spectrum – a “fingerprint” created by the characteristic way a chemical breaks down when exposed to an ionization source.
co-elution – occurs when two chemicals are not sufficiently separated on the GC column and must be separated by some other means, such as mass spectrum.
precursor ion – the ion from a chemical’s mass spectrum that is chosen for further fragmentation during MS/MS.
product ion – the ion from a chemical’s mass spectrum that is chosen from the fragmentation spectrum of the precursor ion.
mass transition – a precursor ion and one of its product ions.
Go to top!
Please contact caitlin.collins@tufts.edu with any questions.